Rapid PNGase F (non-reducing format) (P0711) SDS-PAGE Protocol
To visualize the intact multimeric protein, samples must be prepared in loading buffer without DTT. Use NEB #B7703S, 3X non-reducing SDS loading buffer (use as provided, do not add DTT).
1. Add 5 µl of 3X non-reducing loading buffer per 10 µl of sample containing 2–3 µg of glycoprotein (gel shifts are most noticeable when sample does not exceed 3 µg of protein per lane. However, for heterogeneous glycoproteins the optimal load has to be determined experimentally).
2. Incubate for 5 minutes at 95°C, cool down.
3. Load sample and controls side-by-side on a 10–20% Tris-Glycine gel.
4. Run gel at 130–200V.
5. Remove the gel from the cast and place it in a plastic tray with Coomassie Blue Stain Solution (follow manufacturer recommendations).
6. Record images using a white light trans-illuminator or scanner.
Reducing gels:
For large multimeric proteins (such as IgGs) it is easier to visualize MW shifts of the monomer bands. Prepare NEB #B7703, 3X reducing SDS loading buffer (4 µl of 1.25 M DTT, 130 µl of 3X SDS Loading Buffer).
1. Add 5 µl of 3X reducing loading buffer per 10 µl of sample containing 2–3 µg of glycoprotein.
2. Incubate for 5 minutes at 95°C, cool down.
3. Load sample and controls side-by-side on a 10–20% Tris-Glycine gel.
4. Run gel at 130–200V.
5. Remove the gel from the cast and place it in a plastic tray with Coomassie Blue Stain Solution (follow manufacturer recommendations).
6. Record images using a white light trans-illuminator or scanner.
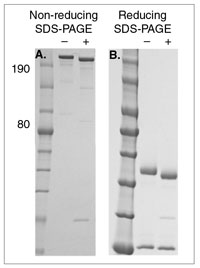
SDS-PAGE of an IgG sample deglycosylated with Rapid PNGase F (non-reducing format) (+) compared with an untreated control (–). Panel A demonstrates non-reducing SDS-PAGE conditions. The quaternary structure of the IgG is preserved, IgG migrates as a high molecular weight band. Panel B demonstrates reducing SDS-PAGE conditions, to help visualizing the complete shift in migration of the IgG heavy chain after deglycosylation with Rapid PNGase F (non-reducing format). Note that the light chain shows no shift (not glycosylated).
